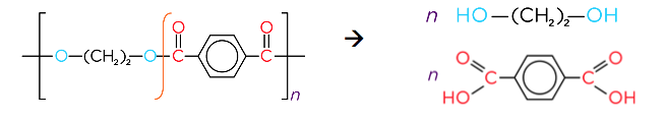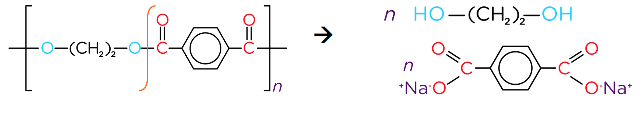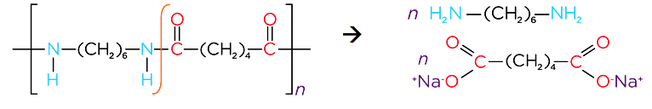A-LEVEL OCR ChEMISTRY NOTES
Polyesters and Polyamides
Condensation Polymers
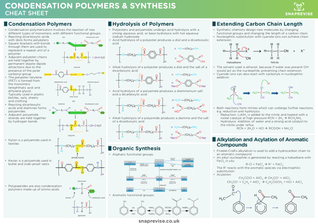
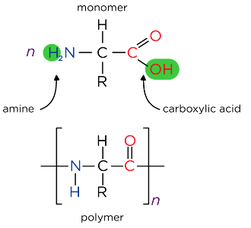
- Condensation polymerisation involves the reaction of two different types of monomers, with different functional groups.
- Reacting dicarboxylic acids with diols forms polyesters
- Square brackets with bonds through them are used to represent a repeat unit of a polymer
- Adjacent polyester chains are held together by permanent dipole-dipole attractions due to the presence of the polar carbonyl group
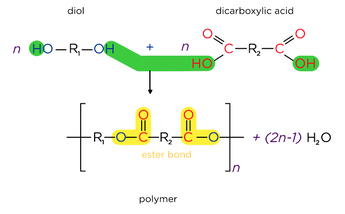
- The polyester terylene (PET) is formed from the monomers terephthalic acid and ethylene glycol. Typically used in plastic bottles, sails, sheets and clothing
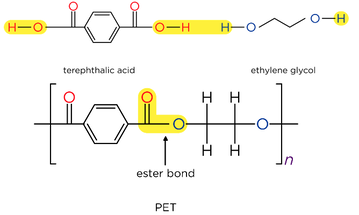
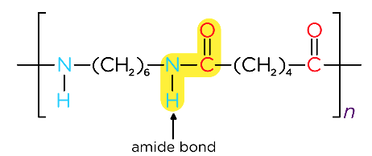
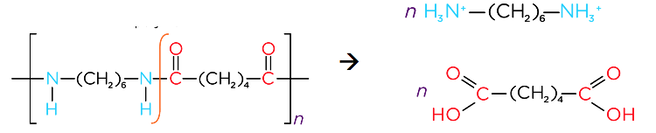
- Reacting dicarboxylic acids and diamines forms polyamides
- Adjacent polyamide strands are held together by hydrogen bonds
- Nylon is a polyamide used in textiles
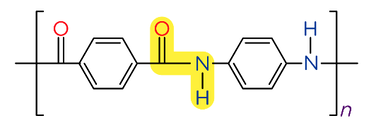
- Kevlar is a polyamide used in bullet and stab-proof vests
- Polypeptides are also condensation polymers made up of amino acids
Hydrolysis of Polymers
- Polyesters and polyamides undergo acid hydrolysis with a strong aqueous acid, or base hydrolysis with hot aqueous sodium hydroxide
- Acid hydrolysis of a polyester produces a diol and a dicarboxylic acid
- Alkali hydrolysis of a polyester produces a diol and the salt of a dicarboxylic acid
- Acid hydrolysis of a polyamide produces a diammonium salt and a dicarboxylic acid
- Alkali hydrolysis of a polyamide produces a diamine and the salt of a dicarboxylic acid