A-LEVEL AQA ChEMISTRY NOTES
organic analysis
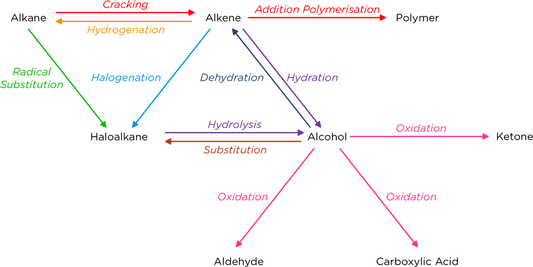
Synthetic Routes
Mass Spectrometry
- A molecular ion (M+) is formed when molecules in a sample are ionised. This can be done using two methods:
- Electron-impact ionisation – the sample is vaporised and bombarded with high energy electrons and an electron is removed
- Electrospray ionisation
- The molecular ion peak (the peak with the highest m/z ratio in the mass spectrum) can be used to determine the relative molecular mass
- Molecular ions are able to break up into fragments during ionisation. This creates a fragmentation pattern on the mass spectrum. Different compounds will produce different fragment peaks
Infrared Spectrometry
- A chemical bond constantly vibrates and can absorb infrared radiation at characteristic wavenumbers
- Particular bonds, and therefore functional groups, can be identified by looking at the frequencies absorbed in a infrared spectrum.
- The fingerprint region is the region of an infrared spectrum below 1500 cm^-1.
- The fingerprint region is largely unique to a specific molecule and allows identification of a molecule by comparison to a database of compounds.
- The bonds present in greenhouse gases (carbon dioxide, methane & water) can absorb infrared radiation that is reflected by the Earth’s surface, contributing to global warming.
- Infrared spectroscopy can also be used to identify impurities. Extra peaks in an infrared spectrum indicate that the sample is not pure.
Test Tube Reactions
- The presence of alkenes can be tested by adding a few drops of bromine water and shaking. Bromine water will go from orange to colourless if alkenes are present, due to addition of bromine across the double bond.
- The presence of haloalkanes can be tested by adding dilute nitric acid and adding silver nitrate. A coloured precipitate is formed.
- Ag^+(aq) + X^-(sq) → AgX(s)
Silver Halide |
Colour |
Haloalkane Present |
Silver Chloride |
White Precipitate |
Chloroalkane |
Silver Bromide |
Cream Precipitate |
Bromoalkane |
Silver Iodide |
Yellow Precipitate |
Iodoalkane |
- Ketones and aldehydes can be distinguished by using Tollens’ reagent or Fehling’s solution
- Tollens’ reagent is made by dilute sodium hydroxide solution to a colourless silver nitrate solution, forming a light brown precipitate. Then dilute ammonia solution is added to dissolve the brown precipitate, forming a colourless solution. Warming the sample with Tollens’ reagent will form a silver mirror if an aldehyde is present.
- Warming the sample with Fehling’s solution will turn the solution from blue to a dark red precipitate if an aldehyde is present
- The presence of carboxylic acids can be tested by adding a metal carbonate. If present, CO2 will be formed, which turns limewater cloudy.
metal carbonate + acid → salt + carbon dioxide + water
- The presence of alcohols can be tested by the addition of acidified potassium dichromate(VI). If a primary or secondary alcohol is present, the orange solution will turn green.