A-LEVEL AQA ChEMISTRY NOTES
Halogenoalkanes
Halogenoalkanes
- Haloalkanes are saturated organic compounds that contain at least one halogen atom, e.g. F, Cl, Br, or I.
- The C-X bond has a permanent dipole due to the large difference in electronegativity between the carbon and halogen atoms. With the electrons closer to the halogen atom.
- The δ- on the carbon atom makes it easily attacked by electron-rich nucleophiles (an electron pair donor that is attracted to electron deficient regions).
- Nucleophilic substitution with hydroxide ions:
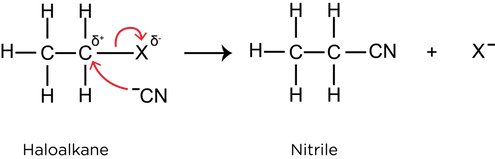
- Nucleophilic substitution with cyanide ions:
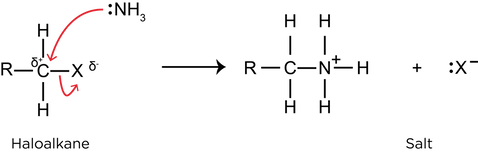
- Nucleophilic substitution with ammonia:
if ammonia is used in excess:
- Going down Group 7, the electronegativity of the halogen atoms decreases, so the polarity of the C-X bond also decreases. We would expect fluoroalkanes to be the most reactive, however iodoalkanes are the most reactive because bond enthalpy decreases down the group. C-I bond is the longest and weakest
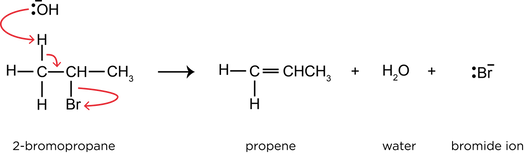
- When a haloalkane is reacted with hydroxide ions under different conditions, an elimination reaction occurs, as the hydroxide ions act as a base.
- 2-bromopropane reacts with potassium hydroxide to form propene, water, and a bromide ion:
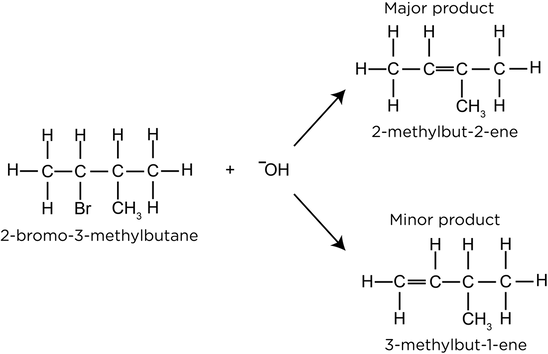
- Major and minor products can form. The major product has more substituted double bonds
- When haloalkanes react with OH- ions, there are two possibilities: substitution or elimination. Dependant on conditions used
Substitute |
Elimination |
|
|
Ozone Depletion
- In the Earth’s atmosphere, there is a layer of ozone (O3) which protects us from the harmful UV radiation produced by the sun
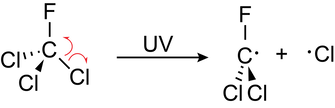
- Chlorofluorocarbons (CFCs) are a type of organic compound that contain chlorine and fluorine atoms. They can diffuse through layers of the atmosphere where they are exposed to UV radiation
- The chlorine radials produced cataluse the decomposition of ozone
Cl• + O3 → ClO• + O2
ClO• + O3 → 2O2 + Cl•
Overall: 2O3 → 3O2
ClO• + O3 → 2O2 + Cl•
Overall: 2O3 → 3O2
- The chlorine radicals are regenerated and act as a catalyst in the breakdown of ozone molecules into oxygen
- Holes in the ozone layer increase the likelihood of skin cancers and sunburns